Please help with some Sec 3 Chemistry Questions
-
Hi all,
Can help to answer the following Chemistry questions? Thanks in advance.
-
Can you try the question yourself first?
-
New generation - just give me the answer cos I am busy dating and playing game.
-
Why not you try doing the question on a fullscape first, then show us your solution. We'll then correct your mistakes from there. Okay?

-
Originally posted by kkhing:
Hi all,
Can help to answer the following Chemistry questions? Thanks in advance.


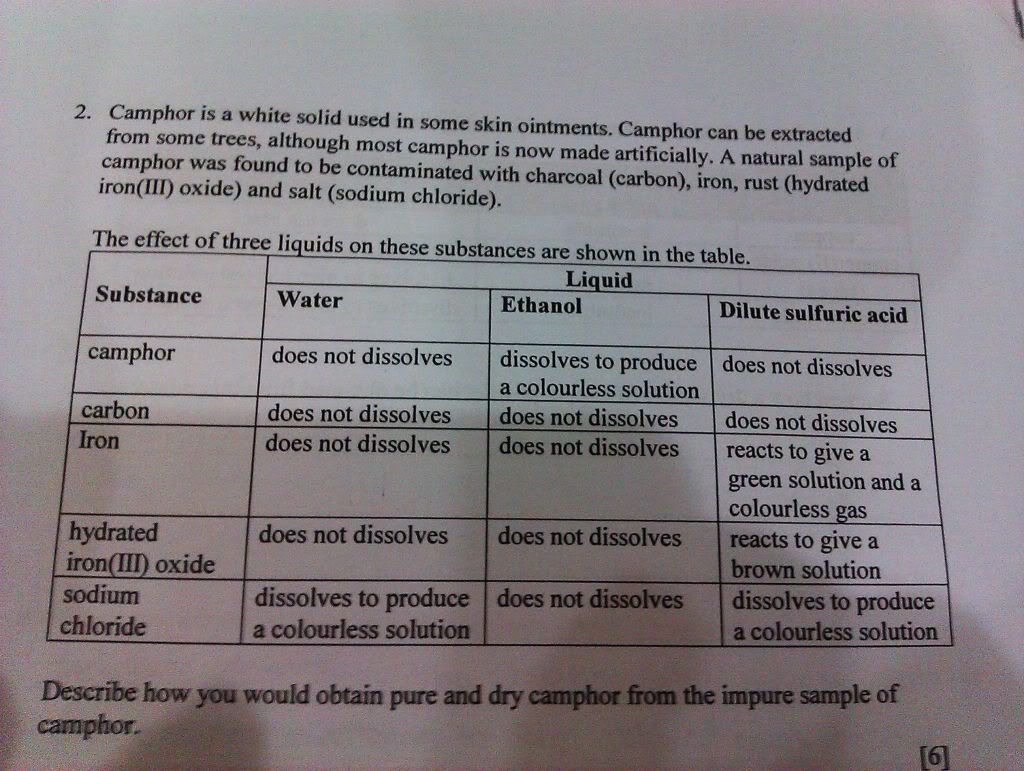
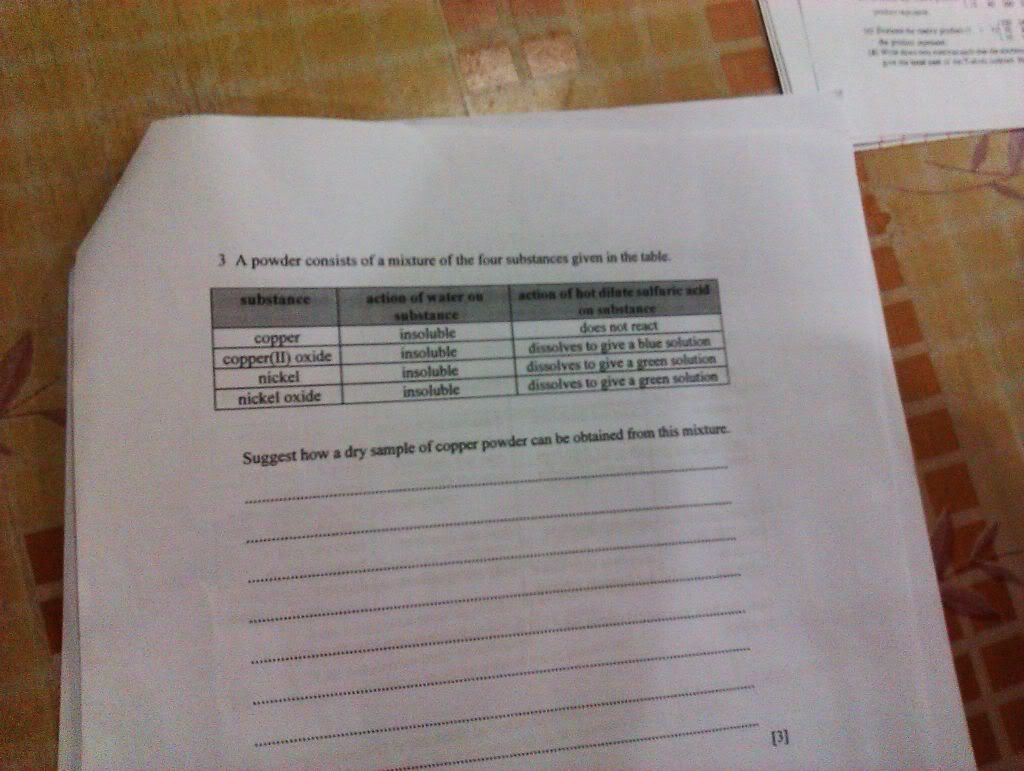
Let me have a go :) 1(a) this one a bit funny how to get it- draw the sugarcane smasher at the shop to collect the juice from the sugar cane? (b) crystallization. heat the solution in an evaporating dish until 1/3 of the original amount of solution is present. place a glass rod into the evaporating dish, if crystals start to form on the glass rod then stop heating it and let the solution cool and crystallize. the sugar crystals will form on the evaporating dish. filter the mixture. finally, open up the filter paper containing the crystals and use another filter paper to dry the sugar crystals. (c) melt the crystals. if a there is an impurity present the melting point will occur in a range that is lower than the melting point of the crystal (if impurity is non-volatile). while if it is pure, it will melt at a definate temperature. (d) it will boil in a range that is above 100 degrees celcius. the crystals acts as an impurity. hence the boiling point will increase and be at a range of 100 degrees celcius. 2) (i write not so detailed now you should be able to do these questions...) dissolve in water to get rid of NaCl, put H2SO4 to get rid or iron and rust, then pour ethanol to get a solution of camphor. then do crystallization to get camphor 3) did u skip school or something -.-