GCE A Level H2 Chem Paper 1
-
Just finished Chem Paper 1!
Some questions.
Question 2
In 1892, Lord Rayleigh made 'atmospheric nitrogen' by removing carbon dioxide, water vapour and oxygen from a sample of air. He found the density of this nitrogen to be 1.2572 g dm^-3 at s.t.p. Chemically pure nitrogen has a density of 1.2505 g dm^-3 at s.t.p.
Which gas present in 'atmospheric nitrogen' caused this discrepancy?
A argon
B helium
C methane
D neonQuery: Answer is A, cos I did this question before. Is there any way to actually calculate out?
Question 15
A student made up a 0.10 mol dm^-3 solution of Ba(OH)2.8H2O which she found in the laboratory cupboard and left the solution in an open beaker. A week later, she returned to the laboratory, used the solution for titration with 0.10 mol dm^-3 HCl and was surprised to discover her titres were lower than expected.
What explains why the values were low?
A Some of the barium hydroxide had reacted with carbon dioxide in the air to form solid barium carbonate.
B Some water had evaporated from the barium hydroxide solution.
C The concentration of HCl was less than the stated 0.10 mol dm^-3.
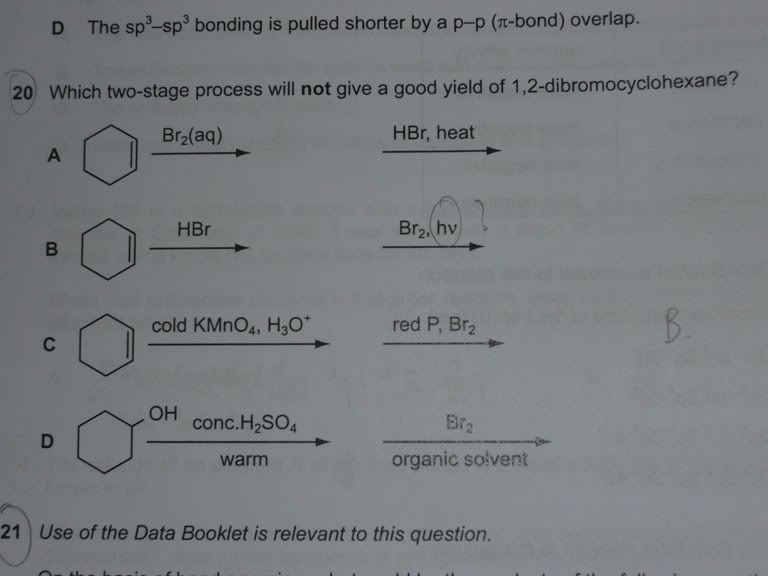
D The crystals had less water of crystallisation than stated.Question 20
-
Originally posted by secretliker:
Just finished Chem Paper 1!
Some questions.
Question 2
In 1892, Lord Rayleigh made 'atmospheric nitrogen' by removing carbon dioxide, water vapour and oxygen from a sample of air. He found the density of this nitrogen to be 1.2572 g dm^-3 at s.t.p. Chemically pure nitrogen has a density of 1.2505 g dm^-3 at s.t.p.
Which gas present in 'atmospheric nitrogen' caused this discrepancy?
A argon
B helium
C methane
D neonQuery: Answer is A, cos I did this question before. Is there any way to actually calculate out?
Question 15
A student made up a 0.10 mol dm^-3 solution of Ba(OH)2.8H2O which she found in the laboratory cupboard and left the solution in an open beaker. A week later, she returned to the laboratory, used the solution for titration with 0.10 mol dm^-3 HCl and was surprised to discover her titres were lower than expected.
What explains why the values were low?
A Some of the barium hydroxide had reacted with carbon dioxide in the air to form solid barium carbonate.
B Some water had evaporated from the barium hydroxide solution.
C The concentration of HCl was less than the stated 0.10 mol dm^-3.
D The crystals had less water of crystallisation than stated.Question 20

Q2. Ans is argon. Reason is only argon (of the 4 choices) is heavier than N2. You can't calculate any values out, since they did not give any additional required info.Q15. Ans is option A. Carbon dioxide is an acidic oxide and thus dissolves in water to form aqueous carbonic acid, H2CO3; which neutralizes (uses up) some of the barium hydroxide alkali.
Q20. Ans is option B. "hv" stands for irradiation with UV light. Yield will be very low since any of the other 11 hydrogens could also be free-radical substituted away by the Br radical.
-
q2) also wrote argon, same reason as you. i think it is because of the fact that it is most dense among the rare gases. since the rare gases are in a very small percentage in atmospheric air, density plays an important part.
q15) A.
eliminate choice B and D, since they did nothing to alter the concentration of OH ions or Ba ions.
since titres lower than expected, it means that vol. of HCl used is lower than expected, it either mean that HCl concentration higher than stated or OH concentation lower than expected.
so eliminate choice C. therefore left choice A.
although HCl reacts with solid barium carbonate, it is reminded that people only use the solution in a titration. so the solids are left out, which resulted in a net loss in OH ions.
as for qsn 20, you are right, its B.
Choice A, C and D specifically forms 1,2-dibromocyclohexane.
choice B depends on electrophilic substitution to sub away the adjacent hydrogen on bromocyclohexane, and isomers may be obtain, thus yield is low.
how did you find the paper generally. i find it very tough..... lost more than 10 marks alr, i think.
-
Thanks for the prompt reply!
Q15, how about option B? Some water evaporated, and hence the concentration is higher? Thus value is less than expected. Is there a link?
Q20, luckily I tikam correctly. Cos I never see "hv" before.
-
qsn 3) Use of the Data Booklet is relevant to this question.
the electronic configurations of four elements are given.
which element will most easily form an isolated gaseous ion with the charge of 3+?
A) 1s2 2s2 2p3
B) 1s2 2s2 2p6 3s2 3p3
C) 1s2 2s2 2p6 3s23p6 3d1 4s2
D) 1s2 2s2 2p6 3s2 3p6 3d5 4s2
__________________________
Q7) A pure sample of N2O4 (l) is introdueced into an evacuated vessel. The vessel, of constant volume, is heated to a constant temperature such that the equilibrium below is established.
2NO2 (g) <=> 2NO (g) + O2 (g)
The value of the pressure p is then found to be 20% greater than if only NO2 (g) were present.
What is the mole fraction, x, of oxygen in this equilibrium mixture?
A. 0.17
B. 0.20
C. 0.33
D 0.40
-
Originally posted by secretliker:
Thanks for the prompt reply!
Q15, how about option B? Some water evaporated, and hence the concentration is higher? Thus value is less than expected. Is there a link?
Q20, luckily I tikam correctly. Cos I never see "hv" before.
concentration higher, but the amount of ions inside the solution will still be the same, as the water which have evaporated are not involved in any reaction. -
>>> choice B depends on electrophilic substitution to sub away the adjacent hydrogen on bromocyclohexane, and isomers may be obtain, thus yield is low. <<<
To be precise, it's free radical substitution, not electrophilic aromatic substitution.
-
Question 7:
2NO2 (g) <=> 2NO (g) + O2 (g)
Initial: 2 0 0
Final 2 - x x x/2
(2 - x + x + x/2) / 2 = 1.2
2 + x/2 = 2.4
x/2 = 0.4
Therefore ans = D.
-
Originally posted by deathmaster:
qsn 3) Use of the Data Booklet is relevant to this question.
the electronic configurations of four elements are given.
which element will most easily form an isolated gaseous ion with the charge of 3+?
A) 1s2 2s2 2p3
B) 1s2 2s2 2p6 3s2 3p3
C) 1s2 2s2 2p6 3s2 3p6 3d1 4s2
D) 1s2 2s2 2p6 3s2 3p6 3d5 4s2
__________________________
Q7) A pure sample of N2O4 (l) is introdueced into an evacuated vessel. The vessel, of constant volume, is heated to a constant temperature such that the equilibrium below is established.
2NO2 (g) <=> 2NO (g) + O2 (g)
The value of the pressure p is then found to be 20% greater than if only NO2 (g) were present.
What is the mole fraction, x, of oxygen in this equilibrium mixture?
A. 0.17
B. 0.20
C. 0.33
D 0.40
Q3. Ans is option C. Scandium. -
q12) Which statement is correct about a reaction for which equilibrium constant is independent of temperature?
A. The rate constants for the forward and reverse reactions do not vary with temperature.
B. the activation energies for both forward and reverse reactions are zero.
C. The enthalpy change is zero.
D. There are equal numbers of moles of reactants and products.
_______________________
q18)
Use of the Data Booklet is relevant to this question.
In the Aromas Red Sands aquifer, the drinking water source for part of California, there are high levels of soluble, toxic chromium (VI) compounds.
Which Compound in the aquifer's sands is most likely to be responsible for the formation of the chromium (VI) compounds from the sparingly soluble chromium (III)-bearing rocks?
A. Al2O3
B CuO
C Fe2O3
D ZnO
_____________________
q31)
Carbon forms double bonds with each of the group VI elements oxygen, sulphur and selenium. In each case, the double bond is polar.
In the molecules carbon dioxide (CO2), carbonyl sulphide (COS) and carbonyl selenide (COSe), the polarities of these couble bonds do not necessarily cancel.
Overall polarity of molecule.
CO2 -- 0
COS ---0.71
COSe --- 0.73
Which factors could account for these observations?
1. The C=S bond is more polar than the C=Se bond.
2. The C=O bond is more polar than the C=S bond
3. The C=Se bond is more polar than the C=O bond.
______________________________
q35.
The followng represents the electronic configuration of both a Group II cation and a Group VII anion.
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6
The radius of the anion is approximately twice that of the cation.
Which reasons explain the difference in size?
1. The cation has a greater nuclear charge than the anion.
2. There is more electron shielding in the anion than in the cation.
3. On forming the anion from its atom, the extra electron repulsion makes the ion much larger.
______________________
-
Question 7:
2NO2 (g) <=> 2NO (g) + O2 (g)
Initial: 2 0 0
Final 2 - x x x/2
(2 - x + x + x/2) / 2 = 1.2
2 + x/2 = 2.4
x/2 = 0.4
Therefore ans = D.
-----------------------------------------------------------
Err answer should be 0.17. A.
correct mol fraction should be wrt your working
0.4/2.4=1/6=0.17
-
Originally posted by surfman:
Question 7:
2NO2 (g) <=> 2NO (g) + O2 (g)
Initial: 2 0 0
Final 2 - x x x/2
(2 - x + x + x/2) / 2 = 1.2
2 + x/2 = 2.4
x/2 = 0.4
Therefore ans = D.
-----------------------------------------------------------
Err answer should be 0.17. A.
correct mol fraction should be wrt your working
0.4/2.4=1/6=0.17
!! Arghs. Too careless. =( -
any answers for 12, 18, 31 and 35?
-
Originally posted by deathmaster:
q12) Which statement is correct about a reaction for which equilibrium constant is independent of temperature?
A. The rate constants for the forward and reverse reactions do not vary with temperature.
B. the activation energies for both forward and reverse reactions are zero.
C. The enthalpy change is zero.
D. There are equal numbers of moles of reactants and products.
_______________________
q18)
Use of the Data Booklet is relevant to this question.
In the Aromas Red Sands aquifer, the drinking water source for part of California, there are high levels of soluble, toxic chromium (VI) compounds.
Which Compound in the aquifer's sands is most likely to be responsible for the formation of the chromium (VI) compounds from the sparingly soluble chromium (III)-bearing rocks?
A. Al2O3
B CuO
C Fe2O3
D ZnO
_____________________
q31)
Carbon forms double bonds with each of the group VI elements oxygen, sulphur and selenium. In each case, the double bond is polar.
In the molecules carbon dioxide (CO2), carbonyl sulphide (COS) and carbonyl selenide (COSe), the polarities of these couble bonds do not necessarily cancel.
Overall polarity of molecule.
CO2 -- 0
COS ---0.71
COSe --- 0.73
Which factors could account for these observations?
1. The C=S bond is more polar than the C=Se bond.
2. The C=O bond is more polar than the C=S bond
3. The C=Se bond is more polar than the C=O bond.
______________________________
q35.
The followng represents the electronic configuration of both a Group II cation and a Group VII anion.
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6
The radius of the anion is approximately twice that of the cation.
Which reasons explain the difference in size?
1. The cation has a greater nuclear charge than the anion.
2. There is more electron shielding in the anion than in the cation.
3. On forming the anion from its atom, the extra electron repulsion makes the ion much larger.
______________________
Instead of me giving the answers outright, deliberately making vague comments is more fun. It's better to let the candidates discuss the answer with each other instead.
Hints for Q12.Temperature change affects position of equilibrium not only because of enthalpy, but also entropy.
Hints for Q18.
Only 1 of the 4 options could act as an oxidizing agent.
Hints for Q31.
Electronegativity increases from left to right, bottom to top, of the Periodic table.
Hints for Q35.
The two ions are isoelectronic (iso = same).
-
Originally posted by deathmaster:
any answers for 12, 18, 31 and 35?
My answers, don't know if correct.12 A (I feel B C D shouldn't be correct)
18 C (assuming not at standard conditions..)
31 D (Option 1)
35 B (Option 1 & 2) -
Originally posted by UltimaOnline:
Instead of me giving the answers outright, deliberately making vague comments is more fun. It's better to let the candidates discuss the answer with each other instead.
Hints for Q12.Temperature change affects position of equilibrium not only because of enthalpy, but also entropy.
Hints for Q18.
Only 1 of the 4 options could act as an oxidizing agent.
Hints for Q31.
Electronegativity increases from left to right, bottom to top, of the Periodic table.
Hints for Q35.
The two ions are isoelectronic (iso = same).
vague indeed...Hints for Q12.
Temperature change affects position of equilibrium not only because of enthalpy, but also entropy.
so i suppose answer is A? since from the boltzman distribution diagram, increasing temperature increases kinetic energy, thus even when enthalpy = 0, rate of reaction still increases, so can eliminate C?
Hints for Q18.
Only 1 of the 4 options could act as an oxidizing agent.
Fe2O3? it has the most positive E. value among the 4 (+0.77), but it is not higher than the +1.33 of Cr(VII).
Hints for Q31.
Electronegativity increases from left to right, bottom to top, of the Periodic table.
So all the options?
Hints for Q35.
The two ions are isoelectronic (iso = same).
so all the options?
-
Originally posted by secretliker:
My answers, don't know if correct.12 A (I feel B C D shouldn't be correct)
18 C (assuming not at standard conditions..)
31 D (Option 1)
35 B (Option 1 & 2)>>> My answers, don't know if correct. <<<
Some right, some wrong. -
Originally posted by deathmaster:
vague indeed...Hints for Q12.
Temperature change affects position of equilibrium not only because of enthalpy, but also entropy.
so i suppose answer is A? since from the boltzman distribution diagram, increasing temperature increases kinetic energy, thus even when enthalpy = 0, rate of reaction still increases, so can eliminate C?
Hints for Q18.
Only 1 of the 4 options could act as an oxidizing agent.
Fe2O3? it has the most positive E. value among the 4 (+0.77), but it is not higher than the +1.33 of Cr(VII).
Hints for Q31.
Electronegativity increases from left to right, bottom to top, of the Periodic table.
So all the options?
Hints for Q35.
The two ions are isoelectronic (iso = same).
so all the options?
Sorry, rushing off for makan. Car waiting for me downstairs.And... like secretliker, you got some right, some wrong.
-
Originally posted by secretliker:
My answers, don't know if correct.12 A (I feel B C D shouldn't be correct)
18 C (assuming not at standard conditions..)
31 D (Option 1)
35 B (Option 1 & 2)
my ans:same as you for 12 and 18.
A for both 31 and 35.
-
my ans: C C B D
for qn 35: since both ions have same electronic configuration, so shielding effect should be the same. so 2 is wrong.
for qn 31: just a guess. but option 3 is wrong as Se is much less electronegative than O since most electronegative element in periodic table is N O F.
-
yup.. qns 31 can't be A should be B.. 35 i put D too.. is 12 D? and 18 confirm C
-
(drinking more chocolate... wait...)
-
based on discussions with friends, IN NO WAY CONFIRMED.
1. B (no. of mol = 1 / 24000, then multiply by Avogadro's constant)
2. A (as said above, only option more molecularly massive than N2)
3. C (data booklet)
4. D (proton number increases, while nucleon number remains constant)
5. C (N-: 1s2 2s2 2p4, remove one to get 2p3, i.e. half filled)
6. C (trivalent N (2 and 4) have a lone pair for dative; the other two have -ve charge, ionic)
7. A (discussed above)
8. D (I2 exist as diatomic molecules, only possibility)
9. A (combustion is an exothermic process)
10. C (heat change must be +ve to absorb heat (cold pack); spontaneity => delG -ve)
11. D (Ka is solely temperature dependent)
12. C (not sure. the fact that LCP doesnt come into play means neither forward nor backward reactions are exo / endothermic. heat change should thus be zero)
13. D (10 halflives later, should halve 10 times, i.e. (1/2)^20)
14. D (1mol of cpd -> 3 mols of Cl-; either Al2Cl6 or PCl3; but liquid, fumes => PCl3)
15. A (discussed above; anyway, B results in no change in titre value; both C and D increase it (if any of them were to happen))
16. C (dispersion forces increases => bp increases; shielding increases=> E.A. decr)
17. B (Fe: [Ar] 3d6 4s2; Fe (II) remove the valence 2)
18. C (as discussed above; highest E0 anyway)
19. A (both CH are sp2 hybridised because 3 sigma bonds, only A possible)
20. B (B results in random subs at any position, i.e. low yield)
21. B (NOT SURE calc using bond energies; B results in most exothermic rxn, i.e. most stable products with respect to reactants)
22. A (duh)
23. A (Cl attached to aryl ring is 'stuck' due to strong bond with partial double bond char)
24. C (NOT SURE from the pattern you're supposed to infer from the given reaction, the O radical part attacks the C atom of epoxyethane, i.e. no O-O bonds so not B or D. A is quite clearly wrong due to the (CH3(CH2)10O) recurring units (10 of them, too O_O ))
25. C (conc H2SO4 for dehyd; NaOH for removing any HCl carry-over)
26. B (homogeneous catalysis with small amt of base or aq KCN)
27. C (phenolic group the only nucleophile to attack electron deficient acyl chloride C)
28. C (+ve iodoform => alcohol part is ethanol. only possibility is C)
29. C (low protonating pH of 2 => both amine groups get protonated to NH3+ groups)
30. D (only non-betaaminocarboxylic acid)
(i hate section Bs for chem mcq)
31. B (C=O bond is v polar. COS overall less polar means C=S bond counters the C=O polarity to a greater extent => C=S more polar than C=Se)
32. D (i put C >_< ) (2 is not even balanced; 3 is nonstandard due to gaseous H2O)
33. B (i put D >_> must have been cock-eyed read left as right) (left-hand electrode is standard hydrogen electrode, so E0cell is E0 of Fe(II)/Fe(III) i.e. +0.77V; right hand electrode higher E0 => reduction occurs => electrons flow to it => it is the positive electrode)
34. B (should be obvious. 3 is wrong because rate constant is 2 x 10^-2. usually cambridge papers dont have this type of cunning thing >_> )
35. D (NOT SURE 1 is definitely correct. (the two are Br- and Sr2+) Sr has more protons => greater nuclear charge => pulls electron cloud tighter; 2 SHOULD be wrong because same electronic config => same shielding effect; 3 looks correct but i think its wrong cos the two have same electronic config, its correct but doesnt explain the difference in size)
36. C (X2 accepts electrons most readily, so it is the strongest oxidising agent => 1 is wrong)
37. A (SN2 mech as inferred from a single activation energy. 1 is correct. 2 is correct cuz bond forming and bond breaking (of C-Hal) occur simultaneously, i.e. bond lengthening; 3 is correct also)
38. B (7 chiral centres is correct; hot acidified KMnO4 cleaves lower left double bond to give ketone on right and carb acid on left, upperright primary OH becomes the other carb acid group, so 2; hot acidified K2Cr2O7 does not oxidise double bond, upperright OH gets oxidised to carb acid, tertiary OH on right side is immune to oxidation; that leaves 3 carbonyl groups)
39. C (aldehyde group, 1 only possible with ketone group; 2 is possible with HOOCCH=CHCO(OCH3)H as the aldehyde, add H2; 3 possible with CH3CHO)
40. A (VERY NOT SURE i saw cross-chain link = lolwut ok 2 and 3 should be correct, as the respective double bonds can break their pi bond to form single bonds with the doubly bonded in the adjacent chains, i.e. C in one with N in another for 2; C in one and C in another for 3; 1 can only form hydrogen bonds, wiki says cross linkages are IONIC OR COVALENT but my friends and I think it refers to any discrete or distinct bond stabilising / bonding side chains in any manner, that would mean H bonds too right? i don't know for sure.....)
if my friends and i are correct, then i get 38! yay haha. hope ya'll did okay too! all the best for remaining papers :)
-
oo Ultima Online posted first, saying 12 is D o.O since teacher i suppose im wrong then >_>
BUT i have to say that... they dont' give any information on phase? the reactants / products could be different phases right. see ah:
carboxylic acid + alcohol <----> ester + water
same no. of reactants, same number of products, but pos of eqm DEFINITELY depends on temperature.... right? haha but i don't know XD maybe i got one less correct then :P
-
this paper is quite hard