A level chem paper 2
-
last question! it was on transition metals.
if i remembered correctly, they gave CuCl2 as the first compound. reacting with conc. HCl gives D which is a yellow-green solution/ppt(i forgot) and heating with Cu and conc. HCl gives E, a colourless solution and adding SO2 to D and water to E gives F, a white compound made up of chlorine and copper only. Both reactions of CuCl2 reacts with HCl in the ratio of 1:1.
i) suggest balanced equations for reactions of CuCl2 which gives D and E
ii) suggest the complex ions in D and E
iii) wad type of reaction is taking place from D to F?
iv) wad type of reaction is taking place from D to F?
v) suggest the compound for F and give its oxidation number.
vi) wad is the shape of the complex ion D? (or E)
yeah. thats about it. the questions are a little jumbled up and based on my memory. maybe someone can correct me. haha. i think there're more questions also.
-
Originally posted by rs rs:
last question! it was on transition metals.
if i remembered correctly, they gave CuCl2 as the first compound. reacting with conc. HCl gives D which is a yellow-green solution/ppt(i forgot) and heating with Cu and conc. HCl gives E, a colourless solution and adding SO2 to D and water to E gives F, a white compound made up of chlorine and copper only.
i) suggest a balanced equation for reactions of CuCl2 which gives D and E
ii) suggest the complex ions in D and E
iii) wad type of reaction is taking place from D to F?
iv) wad type of reaction is taking place from D to F?
v) suggest the compound for F and give its oxidation number.
vi) wad is the shape of the complex ion D? (or E)
yeah. thats about it. the questions are a little jumbled up and based on my memory. maybe someone can correct me. haha. i think there're more questions also.
>>> last question! it was on transition metals.
if i remembered correctly, they gave CuCl2 as the first compound. reacting with conc. HCl gives D which is a yellow-green solution/ppt(i forgot) and heating with Cu and conc. HCl gives E, a colourless solution and adding SO2 to D and water to E gives F, a white compound made up of chlorine and copper only.
i) suggest a balanced equation for reactions of CuCl2 which gives D and E
ii) suggest the complex ions in D and E
iii) wad type of reaction is taking place from D to F?
iv) wad type of reaction is taking place from D to F?
v) suggest the compound for F and give its oxidation number.
vi) wad is the shape of the complex ion D? (or E) <<<
To obtain tetrachlorocopper(II) complex ion :
[Cu(H2O)6]2+ + 4Cl- --> [CuCl4]2- + 6H2O
The VSEPR geometry of this complex ion is tetrahedral. (Cl- ligands are too huge, so you don't get hexachlorocopper(II) ion, which would be octahedral).
SO2 is a reducing agent, and reduces Cu2+ to Cu+, so you get CuCl(s), a white compound. Oxidation state (O.S.) of Cu in CuCl is +1; O.S. of Cl in CuCl is -1.
The reactions involved are redox and ligand displacement reactions.
-
K, going out for dinner/supper liao. By the time I'm back, y'all koon liao. So make sure u guys have enough sleep, wake up refreshed for tmrw's exam paper, and makan enough breakfast so ur brain got glucose and can perform during the exam. Have fun!

-
Originally posted by UltimaOnline:
>>> last question! it was on transition metals.
if i remembered correctly, they gave CuCl2 as the first compound. reacting with conc. HCl gives D which is a yellow-green solution/ppt(i forgot) and heating with Cu and conc. HCl gives E, a colourless solution and adding SO2 to D and water to E gives F, a white compound made up of chlorine and copper only.
i) suggest a balanced equation for reactions of CuCl2 which gives D and E
ii) suggest the complex ions in D and E
iii) wad type of reaction is taking place from D to F?
iv) wad type of reaction is taking place from D to F?
v) suggest the compound for F and give its oxidation number.
vi) wad is the shape of the complex ion D? (or E) <<<
To obtain tetrachlorocopper(II) complex ion :
[Cu(H2O)6]2+ + 4Cl- --> [CuCl4]2- + 6H2O
The VSEPR geometry of this complex ion is tetrahedral. (Cl- ligands are too huge, so you don't get hexachlorocopper(II) ion, which would be octahedral).
SO2 is a reducing agent, and reduces Cu2+ to Cu+, so you get CuCl(s), a white compound. Oxidation state (O.S.) of Cu in CuCl is +1; O.S. of Cl in CuCl is -1.
The reactions involved are redox and ligand displacement reactions.
eh. updated the question! i think it makes a difference. and reactions to give D and E are 2 different reactions. so there's 2 different equations.
-
Ahhh. I wrote [CuCl4]2+ instead of [CuCl4]2-
What a write-po!
-
>>> yeah. thats about it. the questions are a little jumbled up and based on my memory. maybe someone can correct me. haha. i think there're more questions also. <<<
I will need to see the exact question to comment further. I don't want to mislead anyone with potential misinterpretations.In the meantime, how about other candidates share their answers to discuss with rs rs? (secretliker has started the ball rolling.)
-
i wrote ligand exchange reaction. then the shape i wrote square planar, obviously wrong. anyhow hantum one
-
Oh man.... I LOST ALL MY 7 MARKS FOR THIS QN.
Hopefully no mistakes at all for all other qns. I cant rmb the colour of complexes!
-
Originally posted by cubsarecute:
Oh man.... I LOST ALL MY 7 MARKS FOR THIS QN.
Hopefully no mistakes at all for all other qns. I cant rmb the colour of complexes!
u can lose up to 16 marks for this paper, and still get A. -
Redox reaction ah. I go and put oxidation i think don't know why i wrote that. I knew got oxidation and reduction taking place but my brain wasn't workin properly!
i thought the shape was square planar cos my notes [Cu(NH3)4] was square planar and i thought what the heck [Cu(Cl)4] should be the same too but i guess not?
Oh well at least the first 3 qns should be able to get full marks.
-
Can ask something about [CuCl4]2-?
I imagined the 4 Cl to be on a square plane around the Cu (so I also put square planar for the shape), then the top and bottom is H2O?
-
i think the shape IS square planar......
someone prove me wrong?
-
Originally posted by Bellofemme:
i think the shape IS square planar......
someone prove me wrong?
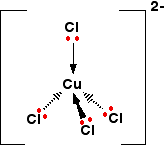
sorry to disappoint all who put square planar.
its a TETRAHEDRAL

the identity for D and F is given by the forummers above already.
so i will give the identity for E : [CuCl4]3-
the Cu here has a oxidation state of +1. U may wonder y it is a colourless soln.
Cu outer shell 4s1... Cu+ means it is 3d10 4s0.. so it has a complete outershell, therefore no d-d transition.. therefore colourless solution.
-
arigatoast wrote :
>>> Cu outer shell 4s1... Cu+ means it is 3d10 4s0.. so it has a complete outershell, therefore no d-d transition.. therefore colourless solution. <<<
Good thinking. Not having any incompletely filled d orbitals, there are no d-d* electron transitions (even though there is still d-d* orbital splitting in presence of ligands due to inter-electron repulsion; but since all the d orbitals are already completely filled, hence no electron transitions possible), hence Cu+ ions are colourless (regardless of ligands).
Non transition metal compounds are colourless *either* because they have no d orbitals at all, or they have completely filled d orbitals. Either way, no d-d* electron transitions, so no colour.
Again, good thinking, arigatoast.
Bellofemme wrote :
>>> i thought the shape was square planar cos my notes [Cu(NH3)4] was square planar and i thought what the heck [Cu(Cl)4] should be the same too but i guess not? <<<
If there are only 4 ligands, naturally the geometry would be tetrahedral (I always advise my students never to memorize without understanding. Unfortunately, due to manpower and time constraints, JCs have little choice but to instruct their students to memorize first as necessity, understand later as luxury).The reason why tetraaminecopper(II) ion is square planar, is because the real name is tetraaminediaquocopper(II) ion. Meaning, it's not [Cu(NH3)4]2+, but it's really actually [Cu(NH3)4(H2O)2]2+.
So the 4 ammonia ligands occupy the corners of the square planar, while the 2 water ligands occupy the top and bottom of the octahedral.
So why do we call it square planar instead of octahderal? Ideally, you should elaborate to say it's both, by means of a labelled 3D drawing, and a brief description (see preceeding paragraph).
--------------------------------------------------
Now that you 'A' level folks are gradudating, wanna consider being an MOE teacher? It's a decent respectable job that has value in these harsh economic times. Give it some consideration.
-
Originally posted by UltimaOnline:
Now that you 'A' level folks are gradudating, wanna consider being an MOE teacher? It's a decent respectable job that has value in these harsh economic times. Give it some consideration.
what are the requirements to be a teacher? i mean the academic requirements.how do you get moe scholarship? is it tough like the other public service scholarships?
and when do you stop ur education for nie diploma? after honours or masters?
i may take history if i can't get into smu or nus biz.....
-
Originally posted by UltimaOnline:
Now that you 'A' level folks are gradudating, wanna consider being an MOE teacher? It's a decent respectable job that has value in these harsh economic times. Give it some consideration.
Leading them into an abyss

Teachers are respectable, but the job scope extends far beyond just teaching.

Lots of admin stuff to do as well.
-
Originally posted by eagle:
Leading them into an abyss

Teachers are respectable, but the job scope extends far beyond just teaching.

Lots of admin stuff to do as well.
C'mon Eagle. If everybody thought that way, who would be there to teach our children? Every job has its pros and cons. And taking into consideration the economic downturn, being an MOE teacher has greater stability and security as compared to banking and finance. -
Originally posted by deathmaster:
what are the requirements to be a teacher? i mean the academic requirements.how do you get moe scholarship? is it tough like the other public service scholarships?
and when do you stop ur education for nie diploma? after honours or masters?
i may take history if i can't get into smu or nus biz.....
You have to check with MOE website, and if you've lots of questions, you can go ask the recruitment staff at MOE HQ (Buona Vista MRT).If by 'MOE Scholarship', you mean they pay for your University education (right after your 'A' levels), then :
If overseas University, I'm not sure if they even offer such scholarships. They probably do not.
If local NIE (NTU) degree, then it's the BSc/BA degree in (subject) with diploma in Education. This is actually not a 'scholarship', but a contract/bond.
However, if you sign the contract/bond and you receive MOE sponsored University education in NIE (NTU), you can only teach primary school, at least for the first few years.
If you want to teach Secondary school or JC, you need to pay for your own University (eg. NUS) degree in your teaching subject (eg. History, Physics, etc)
(That's the last I heard anyway, every year there are policy changes).
You can stop at any level, Bachelors', Honors', Masters', Doctorate. Your pay will take your degree level into consideration.
Any further questions? But do visit the MOE website yourself, most of your queries should be answered there.
-
Originally posted by UltimaOnline:
C'mon Eagle. If everybody thought that way, who would be there to teach our children? Every job has its pros and cons. And taking into consideration the economic downturn, being an MOE teacher has greater stability and security as compared to banking and finance.
Must give them actual idea of job scope first mah
My gf went in with the idea that only teaching and creating materials was heavily involved, which wasn't true.
-
Originally posted by UltimaOnline:
If there are only 4 ligands, naturally the geometry would be tetrahedral (I always advise my students never to memorize without understanding. Unfortunately, due to manpower and time constraints, JCs have little choice but to instruct their students to memorize first as necessity, understand later as luxury).
The reason why tetraaminecopper(II) ion is square planar, is because the real name is tetraaminediaquocopper(II) ion. Meaning, it's not [Cu(NH3)4]2+, but it's really actually [Cu(NH3)4(H2O)2]2+.
So the 4 ammonia ligands occupy the corners of the square planar, while the 2 water ligands occupy the top and bottom of the octahedral.
So why do we call it square planar instead of octahderal? Ideally, you should elaborate to say it's both, by means of a labelled 3D drawing, and a brief description (see preceeding paragraph).
Hey there I know I'm a bit lag to join in the discussion only now, but I was puzzled when I read this--is [CuCl4]2- really tetrahedral instead of square planar? When I did the paper I was quick to think 'tetrahedral' at first but I recalled my notes giving it as an example of a 'square planar' complex so that's what I wrote. And when I checked back after the exam, I found out I was right about my notes:

But as arigatoast showed above, the diagram from chemguide.co.uk shows the complex as tetrahedral. So are my notes wrong (in which case I'll have to let my teachers know haha) or is there a possibility that both answers would be accepted somehow?
Sad to have recalled the wrong answer correctly :(
-
Oh and can anyone confirm that the identity of E is [CuCl4]3- as arigatoast mentioned above instead of [CuCl2]- and whatever the answer is explain why please? I put [CuCl4]3- too hmm. Thanks!
-
hmm..[CuCl2]- looks possible, but what i think [CuCl4]2- is reduced to [CuCl4]3- w/o any lost of Cl atoms.
-
-
Ok, I've checked it out, with multiple high-level reliable sources. I know you guys are gonna hate the findings I'm about to reveal. (I actually love it. But then, because SEAB-Cambridge might not be as open-minded as to see the bigger picture, meaning in effect you guys might still lose your marks even though your answer is arguably correct, so I can understand and sympathize, that you might not find this quite as fascinating as it really should be. Lastly, there's the matter of the ratio of ions involved. If this is specified in the actual question, that those are clues (that you ought to have taken into consideration) leading to only 1 acceptable answer per question part, in which case you can't say SEAB-Cambridge wasn't fair to you).
[CuCl2]- or dichlorocopper(I) anion exists, and is linear geometry.
But [CuCl4]3- or tetrachlorocopper(I) ion exists too, and can exist in either tetrahedral geometry, or octahedral & square planar geometry if it's [Cu(Cl4)(H2O)]3-. 6 ligands form an octahedral geometry, the Cl- ligands occupy square planar while the H2O ligands occupy top and bottom.
[CuCl4]2- or tetrachlorocopper(II) ion exists too, and can exist in either tetrahedral geometry, or octahedral & square planar geometry if it's [Cu(Cl4)(H2O)2]2-). 6 ligands form an octahedral geometry, the Cl- ligands occupy square planar while the H2O ligands occupy top and bottom.
Since all 5 of the above ions exist, arguably SEAB-Cambridge should accept more than one possible answer for at least some of the questions. But whether they do, is another matter altogether. Also, I didn't actually read the exact phrasing of the question itself, and if the SEAB-Cambridge question specified the ratio of Cl- ions to the metal cation in each case, then there may only be 1 acceptable answer for each question part, and you can't say SEAB-Cambridge is being unfair to you.
A couple of points (this may go against how you're feeling now, but Chemistry is really about learning how beautiful the Universe is, not about scores/marks/points and hollow paper qualifications. So take this as a post-course continuing journey of exploration and wonder).
The more (dative aka coordinate) bonds a central metal ion can form with ligands (electron donors), the happier it will be. Simply because more bonds formed results in a more exothermic process, which as far as Gibbs Free Energy is concerned, is certainly a good, feasible thing. True, there might be a slight decrease in entropy as a result (which is not a good, feasible thing), but usually the favourable enthalpy change outweighs the unfavourable entropy change. There is also electronic stability to consider, of course.
You should be aware that Be2+ ion, being in period 2, does not have empty 3d orbitals required to expand its octet. Consequently, any complex ions formed with Be2+ has to be tetravalent or 4 bonds, ie. tetrahedral geometry.
In that case, why would Cu+ or Cu2+ want to just form CuCl or CuCl2 and stop there? Wll there's obviously the matter of molarity and availability to consider. If there's enough Cl- ions available, it makes sense that you would want to get [CuCl2]- ion and (adding even even more Cl- ions) [CuCl4]3- ion, and for Cu2+ ion, you would obtain [CuCl4]2- ion.
But if the more bonds formed the merrier, then why stop at 4 Cl- ion ligands? Why not 6 Cl- ligands? Because Cl being in period 3, is too huge to have 6 Cl- ligands crowding around the central metal ion. There would be too much electronic and steric repulsion.
So we only get 4 Cl- ligands? Then obviously, we would get a tetrahedral geometry, wouldn't we? (Notice the JC notes chillax posted above, like all JCs' notes, are inadequate in that they just expect students to memorize without understanding, without proper explanation. Typical JC stuff.)
But compare the H2O molecule to Cl- anion. H2O is much smaller (for 2 reasons - Cl- is in period 3 and it is an anion too). Hence, the [Cu(H2O)6]2+ and [Cu(H2O)6]+ ions exist (though the process is not as exothermic as if Cl- ligands were used, hence ligand displacement or ligand exchange takes place).
Which means that even in tetrachlorocopper(I) and tetrachlorocopper(II) ions, it is possible, under different conditions, that water molecules might squeeze in between the Cl- ligands, and you would get the octahedral geometry. 6 ligands form an octahedral geometry, the Cl- ligands occupy square planar while the H2O ligands occupy top and bottom. (notice that the JC notes don't explain this? tsk tsk.)
The reason why Cl- ligands occupy square planar, is because they're much larger, bring greater electronic and steric repulsion as compared to the smaller H2O ligands, and hence square planar for the 4 Cl- ligands would be furthest apart and the most stable, while the smaller H2O ligands occupy top and bottom.
That being said, the tetrahedral [Cu(Cl)4]2- and [Cu(Cl)4]3- complex ions also exist, just as the 'square planar' (octahedral, really) [Cu(Cl4)(H2O)2]2-) and [Cu(Cl4)(H2O)2]3-) complex ions also exist.
Delightful, ain't it?

 We Love Chemistry
We Love Chemistry 
-
Thanks UltimaOnline for the thorough explanation! In fact I did find that interesting and wish at least a small paragraph had been in my notes! I suspected that it was the water ligands that caused certain complexes to become square planar but with 2 rows in the notes, each with complexes having 4 ligands (and no water ligands) but having different stereochemistries I was bent on just trusting what I saw :(
As for the question's specifications, I only remember it saying that CuCl2 reacted with Cu metal and HCl (no limiting amount specified) but it also said that the each mole of CuCl2 reacts with 2 moles of HCl. Under such specifications, I believe getting [CuCl2]- should definitely work, but I also managed to balance the equation for getting [CuCl4]3-, assuming the CuCl2 did only react with 2 moles of HCl whereas the Cu metal reacted with an additional 4 moles of HCl:
CuCl2 + Cu + 6HCl --> 2[CuCl4]3- + 6H+
Is my equation correct conceptually? And did I make the wrong assumption that CuCl2 only reacted with 2 moles of HCl whereas Cu reacted with the remaining 4 moles?
There's also the possibility I misread and totally forgot if there were specific mole ratio specifications that would determine specific answers...! Anyone out there remember (and free enough to respond :p)?