[Chemistry] Questions again
-
Answer this first: Are covalent compounds soluble in water?
Then answer these:
Why then, is HCl gas able to dissolve in water to form hydrochloric acid?
SO3 able to form sulphuric acid when dissolved in water?
Ammonia able to dissolve in water to form the ever-common aqueous ammonia?
Chlorine gas and ammonia gas not being able to be collected by downward displacement of water?
Are these just exceptions in chemistry concepts?
Next, titration.
Which do you put, acid or alkali, into the burette and pipette(which ends up in the conical flask)
What colour does indictor phenolphthalein turn in neutral solutions? I know it turns pink in alkalis, and colourless in acids.
Third, period III compounds (or is it aluminium chloride only?).
Aluminium chloride is an ionic compound when solid. But then, why does it turn into a covalent compound when it's a gas? Or have I ran into an unreliable source which gives false info?
Does it apply to period III chlorides only?
Fourth, sublimation.
In the 'O' Level syllabus, which substances that sublime are we required to know?
Ammonium XXX (chloride?)
Carbon dioxide
Iodine
Anhydrous aluminium chloride
Anhydrous iron (III) chloride
Right, this doesn't stop here. I'll need a lot of help on these things
Thanks for answering my doubts. I appreciate it :D
And hey, I'll add more when I think of somemore.
-
Originally posted by Garrick_3658:
Answer this first: Are covalent compounds soluble in water?
Then answer these:
Why then, is HCl gas able to dissolve in water to form hydrochloric acid?
SO3 able to form sulphuric acid when dissolved in water?
Ammonia able to dissolve in water to form the ever-common aqueous ammonia?
Chlorine gas and ammonia gas not being able to be collected by downward displacement of water?
Are these just exceptions in chemistry concepts?
Next, titration.
Which do you put, acid or alkali, into the burette and pipette(which ends up in the conical flask)
What colour does indictor phenolphthalein turn in neutral solutions? I know it turns pink in alkalis, and colourless in acids.
Third, period III compounds (or is it aluminium chloride only?).
Aluminium chloride is an ionic compound when solid. But then, why does it turn into a covalent compound when it's a gas? Or have I ran into an unreliable source which gives false info?
Does it apply to period III chlorides only?
Fourth, sublimation.
In the 'O' Level syllabus, which substances that sublime are we required to know?
Ammonium XXX (chloride?)
Carbon dioxide
Iodine
Anhydrous aluminium chloride
Anhydrous iron (III) chloride
Right, this doesn't stop here. I'll need a lot of help on these things
Thanks for answering my doubts. I appreciate it :D
And hey, I'll add more when I think of somemore.
>>> Are covalent compounds soluble in water? <<<Some are, some are not. Case by case basis. Even those that are soluble, may be soluble for different reasons (eg. hydrogen bonding, ion-dipole interactions, etc).
>>> Are these just exceptions in chemistry concepts? <<<
No, these are not "exceptions" in the sense that they are anomalies of nature or the laws of Chemistry; rather, the problem (why they seem to be so) lies only in that 'O' level Chemistry (like 'O' level any subject) is much oversimplified to general principles that are really for the sake of convenience and simplification. It's not that these principles or concepts are wrong or flawed, just that they do not (by deliberate oversimplification design) fully encompass the proper mechanisms at a level of any significant depth, required to fully understand various phenomena in Chemistry, such as the ones brought up by Garrick.
An illustration of this would be the H-Cl example (see below). This is already a simplified explanation, but the fact remains that 'O' level students are simply not equipped (ie. based on what their schools taught them) to understand the explanation to any satisfactory depth.
>>> Why then, is HCl gas able to dissolve in water to form hydrochloric acid? <<<
Because HCl ionizes (ie. electron transfer, bond pair on H-Cl becomes lone pair on Cl, forming H+ and Cl- ions) to form H+ and Cl- (and this occurs only in water because of reasons indicated at the end of this paragraph), and it is these ions, not HCl itself, that is soluble in water, due to favourable ion-cipole interactions and hence theromdynamic stability in the various states or phases (considering both enthalpy and entropy in the solution process, which is the sum of lattice dissociation process and solvation process).
So Garrick, the bottomline is, endure just a bit more, you'll understand all of these questions you've asked today, when you study 'A' level Chem in just a couple more months time. For next week's exam paper, simply work with what you're taught at the simplified 'O' level stage, it will suffice for the paper itself.
>>> Aluminium chloride is an ionic compound when solid. But then, why does it turn into a covalent compound when it's a gas? Or have I ran into an unreliable source which gives false info? <<<
Even at lower temperatures, aluminium chloride is largely a (polar) covalent compound, with some ionic character (you'd be penalized if you say its "ionic with covalent character", because it's the other way around in the case of aluminium chloride). At lower temperature, its structure is similar to an ionic lattice solid (even though it it still not considered an ionic compound).
At higher temperatures approximately 180 - 190°C (depending on the pressure), it exists as a dimer, Al2Cl6. At such temperature, it exists as a simple covalent molecular compound, with dative bonds donated by chlorine to the electron deficient Al (to achieve a stable octet).
At even higher temperatures, it breaks up into individual AlCl3 molecules.
Solid aluminium chloride doesn't conduct electricity at room temperature because the ions aren't free to move. Molten aluminium chloride (only possible at increased pressures) doesn't conduct electricity because there aren't any ions any more.
All of these are not expected to be understood or learnt by the 'O' level candidate. I've given this info only because Garrick asked about this compound.
Bottomline : just endure a couple more months, you'll soon get to enjoy understanding all these at a deeper, more accurate, and more truthful level, when you study these in the JCs or in the Polytechnics.
>>> In the 'O' Level syllabus, which substances that sublime are we required to know? <<<
Pretty much those which you listed. Read the data given in your exam question carefully. The data (eg. melting point, boiling point, solubility, conductivity, etc) will hint to you as to the nature of the substance, even if it's something seemingly 'new' that you've not come across in your course of study. This is called "examining the candidates' ability to apply what he/she has learn to new situations/scenarios, new compounds, new problems". Every exam, including the 'O' levels and 'A' levels, will have such questions.
Enjoy!

-
Oh, on a related note, fyi, the fact that aluminium oxide is amphoteric (ie. both acidic and basic) is related to the fact that aluminium oxide is polar covalent (ie. both covalent and ionic character).
Which in turn, is related to the high charge density of the 3rd period, tripositive ionic nature of aluminium.
A bit over the top for 'O' level students, but I thought I'd just mention it as a btw (by the way) fyi (for ur info), since Garrick brought it up.
-
I've thought over this a bit, and with regards to the solubility of certain covalent gases, I figured I could simplify it further to be easily understood at 'O' levels, if you think of it this way :
>>> Answer this first: Are covalent compounds soluble in water?
Then answer these:
Why then, is HCl gas able to dissolve in water to form hydrochloric acid?
SO3 able to form sulphuric acid when dissolved in water?
Ammonia able to dissolve in water to form the ever-common aqueous ammonia?
Chlorine gas and ammonia gas not being able to be collected by downward displacement of water?
Are these just exceptions in chemistry concepts? <<<
Simplified 'O' level explanation : covalent gases which are acidic (eg. SO3, HCl, etc) or basic (eg. NH3), will IONIZE (ie. be converted to ions) when dissolved in water. And as you know, ions are (mostly) soluble in water.
('O' level students are probably thinking, "There, that wasn't so tough to say, was it? He needn't have gone into an 'A' level lecture in the 1st place... hmmmph!" To that I will reply, "My bad.")
>>> What colour does indictor phenolphthalein turn in neutral solutions? I know it turns pink in alkalis, and colourless in acids. <<<
The pH range of phenolphthalein is 8.3 to 10.0; meaning it is colourless in pH less than 8.3, and pink in pH greater than 10.0; in between 8.3 and 10.0, it is a mixture of colourless & pink (ie. very pale pink). So to answer your question, Garrick, in pH 7 (neutral), it is colourless.
Any further qns?
-
Thanks a trillion, UltimaOnline :D
What about these? :D
Next, titration.
Which do you put, acid or alkali, into the burette and pipette(which ends up in the conical flask)
I assume, acid in the burette and alkali (with indictor phenolphthalein) added in the conical flask? Stupid textbook tells me the other way round. Colourless to colourless, so what is that damn book trying to find?
Fourth, sublimation.
In the 'O' Level syllabus, which substances that sublime are we required to know?
Ammonium XXX (chloride?)
If they want me to draw aluminium chloride (or any period III chloride) in solid state (a.k.a. r.t.p., I should draw an ionic compound right? What if (or will they) ask me to draw it in gaseous state?
Thanks again!
-
Originally posted by Garrick_3658:
Thanks a trillion, UltimaOnline :D
What about these? :D
I assume, acid in the burette and alkali (with indictor phenolphthalein) added in the conical flask? Stupid textbook tells me the other way round. Colourless to colourless, so what is that damn book trying to find?
If they want me to draw aluminium chloride (or any period III chloride) in solid state (a.k.a. r.t.p., I should draw an ionic compound right? What if (or will they) ask me to draw it in gaseous state?
Thanks again!
>>> I assume, acid in the burette and alkali (with indictor phenolphthalein) added in the conical flask? Stupid textbook tells me the other way round. Colourless to colourless, so what is that damn book trying to find? <<<You are correct. See :
http://www.gcsescience.com/aa29.htm
>>> If they want me to draw aluminium chloride (or any period III chloride) <<<
At 'O' levels, it could only be either trichloroborane (BCl3) or aluminium chloride (AlCl3), both of which are actually (in truth) covalent. However, for the purpose of (simplified to the extent of being technically inaccurate) 'O' Levels, the required answer (dot-&-cross diagram) is an ionic structure.
>>> What if (or will they) ask me to draw it in gaseous state? <<<
This gets a little trickier. IF they actually even ask this question at 'O' Levels (which is doubtful), than the question will likely give you additional info (particularly, the low melting & boiling points; and possibly, a molar mass or relative molecular mass of 267g), which will indicate that it is :
#1 a covalent compound (since it has relatively low melting and boiling points)
#2 exists as a dimer Al2Cl6 (since it has a molar mass of 267g).
In which case (IF this really comes out in the 'O' levels, then... you owe me a treat... juz kidding lah, just come for my 'A' levels Chem tuition next year can liao...


 ), then you should draw it as a covalent compound.
), then you should draw it as a covalent compound.However, because this requires dative bonding, which is not covered in the 'O' levels, I expect that at the most, the 'O' level version of this question won't actually require you to draw the compound, but merely to state that, based on the data given in the question, you should be able to deduce that it is :
#1 a covalent compound (since it has relatively low melting and boiling points)
#2 exists as a dimer Al2Cl6 (since it has a molar mass of 267g).
FYI then, this is the structure of the covalent molecule Al2Cl6 :
(note : the 2 arrows represent dative (ie. donated) bonds, which means instead of a cross-&-dot, those bonds are actually cross-&-cross, if you use crosses to represent electrons from chlorine)
( image made in China : http://dl.zhishi.sina.com.cn/upload/99/05/44/1070990544.5378517.JPG )
(Pssst, speaking of made in China... here's the chemical structure of melamine... in case it comes out for your 'O' level or 'A' level exams)
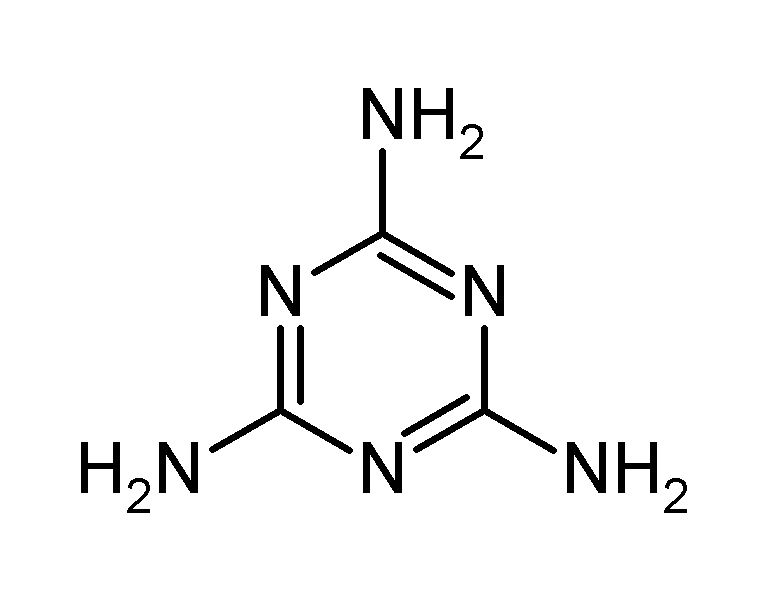
(Image hosted by http://upload.wikimedia.org/wikipedia/commons/3/36/Melamine.png )
>>> Thanks a trillion, UltimaOnline :D <<<
 Most Welcome
Most Welcome 