Electrophilic substitution of phenol
-
Phenol + dil. HNO3 (aq) ->
at room temperature.
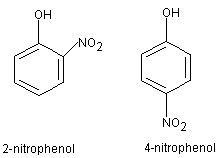
We all know that the substituent -NO2 could be substituted on the 2nd, 4th, or 6th position.
Usually, we write the mole ratio of 2-nitro and 4-nitrophenol as 1:1.
But since 2-nitrophenol is the same as "6-nitrophenol", shouldn't the mole ratio of 2-nitrophenol:4-nitrophenol be 2:1?
-
Originally posted by secretliker:
Phenol + dil. HNO3 (aq) ->

at room temperature.
We all know that the substituent -NO2 could be substituted on the 2nd, 4th, or 6th position.
Usually, we write the mole ratio of 2-nitro and 4-nitrophenol as 1:1.
But since 2-nitrophenol is the same as "6-nitrophenol", shouldn't the mole ratio of 2-nitrophenol:4-nitrophenol be 2:1?
>>> But since 2-nitrophenol is the same as "6-nitrophenol", shouldn't the mole ratio of 2-nitrophenol:4-nitrophenol be 2:1? <<<
Your train of thought is correct, but... (read on below)
>>> Usually, we write the mole ratio of 2-nitro and 4-nitrophenol as 1:1. <<<
This is not correct. We (ie. you, in the exam) only write it that way to show that a MIXTURE of ortho(or 2)-nitrophenol and para(or 4)-nitrophenol is obtained. It is NOT meant to be a stoichiometric value.
There is another factor to consider - steric hinderance would result in the para position to be favoured by the incoming electrophile, rather than the ortho positions.
In practice, the exact ratio of ortho and para isomers is certainly not 1:1 (at 'A' levels, you don't need to know the exact ratio, you only need to say a mixture of both isomers is obtained).
-
Oh ok thanks!