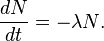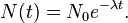Help in A level physics questions (nuclear physics.)
-
1)The radioactive isotope 232,92U (meaning mass number =232, proton number = 92) emits alpha particles with an energy of 5.30MeV and has a half life of 74years. For a sample containing 1.30 X 10^24 atoms, calculate the maximum theoretical power output. ( ans : 3300W)
2)A patient is to be given an injection of iodine-131 in investigation of her blood volume.Sample A is injected into the patient while sample B is set on one side.
Each sample has an initial activity of 8000 Bq and iodine-131 has halflife of 8 days.
After 24days, total activity of iodine-131 remaining in the patient body is estimated to be 400 Bq.
Calculate the biological half life of iodine-131 in the body of the patient( ans : 18 days)
Thx to those who can help. -
1)The radioactive isotope 232,92U (meaning mass number =232, proton number = 92) emits alpha particles with an energy of 5.30MeV and has a half life of 74years. For a sample containing 1.30 X 10^24 atoms, calculate the maximum theoretical power output. ( ans : 3300W)
Check the answer again... I got 330W
Solution:
Power = change in N * 5.3MeV / change in T (in seconds) = dN / dT * 5.3MeVThe largest value of dN/dT occurs at N = 1.3e24 since
so, subsitutiting N, and lambda = ln 2 / (74*365*24*3600s), and 1eV = 1.602e-19 W,
answer to 2 sf is 330W
the negative sign is removed since it shows N2 (final) - N1 (initial)
-
2)A patient is to be given an injection of iodine-131 in investigation of her blood volume.Sample A is injected into the patient while sample B is set on one side.
Each sample has an initial activity of 8000 Bq and iodine-131 has halflife of 8 days.
After 24days, total activity of iodine-131 remaining in the patient body is estimated to be 400 Bq.
Calculate the biological half life of iodine-131 in the body of the patient( ans : 18 days)I think some values somewhere is wrong... I think maybe initial should be 1000Bq instead of 8000Bq... Recheck... Anyway... my solution:
Let N0 = 8000
N(t) = 400
so exp(-λt) = 400/8000 = 1/20
-λt = ln (1/20) = -2.99573, where t = 24 daysso λ = 0.124822, where λ = ln 2 / half-life
Thus, ln 2 / half-life = 0.124822
half-life = 5.6 days